
The Drug Development Process is divided into different steps:
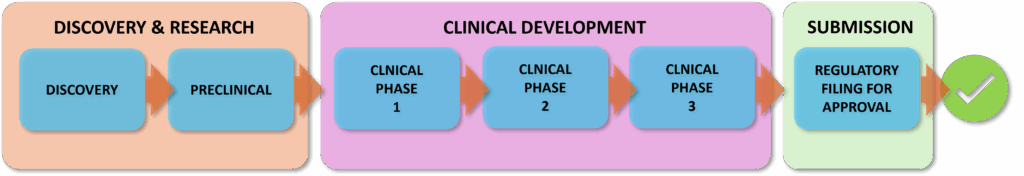
Drug Discovery & Research is a complex and time-consuming process that involves identifying and developing potential new medicines. The journey begins with target identification, where scientists pinpoint specific biological targets (such as e.g. proteins) linked to a particular disease. Once a target is identified drug discovery starts. Vast compound libraries are screened or computational methods (also using nowadays Artificial Intelligence) are used to discover molecules that interact with these targets. Promising candidates undergo rigorous preclinical testing, including cell and animal studies to assess their safety, efficacy and mechanism of action. This stage is crucial to identify potential side effects and optimize the drug’s properties before progressing to clinical trials.
- Discovery & Development: Research for a new drug in the laboratory
- Preclinical Research: Drugs undergo laboratory and animal testing to answer basic questions about safety
Clinical Development is the process of bringing new medicines, devices or treatments through scientific experiments to the market. It involves conducting clinical trials on humans to evaluate the effectiveness and safety of these new products. Typically, there are 3 phases until approval (I, II, III) with an increasing number of participants. Phase IV is after product launch. Usually, the primary goals of a trial in a clinical phase are:
- Clinical Phase 1 trials: Dose-ranging on healthy volunteers or patients for safety
- Clinical Phase 2 trials: Testing of drug on participants to assess efficacy and side effects
- Clinical Phase 3 trials: Testing of drug on participants to assess efficacy, effectiveness and safety
- (Phase IV: post marketing surveillance in public) – this phase is excluded from this simulation)
SUBMISSION AND REVIEW BY REGULATORY AUTHORITIES:
Successful Phase 3 clinical trials are a pivotal milestone in drug development, as they demonstrate the therapy’s safety and efficacy in a large patient population. These positive results pave the way for the submission of a New Drug Application (NDA) to key regulatory bodies worldwide, such as the FDA (U.S.), the EMA (European Union), and other national health authorities.
The NDA is a comprehensive dossier containing an exhaustive compilation of data. This includes detailed findings from preclinical studies, which assess the drug’s initial safety and biological activity, as well as the complete results from all phases of clinical trials, culminating in the critical Phase 3 data.
Upon submission, regulatory bodies undertake a rigorous assessment of the NDA. Their meticulous review scrutinizes every aspect of the data to evaluate the drug’s benefits against its risks. If the review is favorable, the drug will receive regulatory approval, allowing it to enter the market and become available to patients.
In this business simulation, your primary objective is to gain regulatory approval for your drugs. The more drugs you successfully bring through the drug development process to market, the greater your revenues, which will drive your share price and business success.
There have been a lot of studies regarding success rates of drug development phase in the last years (e.g. Schumacher, Hindler et al., Pammolli et. al, Dowden and Munro, Wong Siah, Lo, Young et al., …) looking at various companies, therapeutic areas and projects. The success rate for Clinical Phase 1 is between 44%-80%, for Clinical Phase 2 between 19%-70% and for Clinical Phase 3 between 31%-69%, heavily dependent on therapeutic areas. Overall likelihood of success for clinical development (from Phase 1 to Approval) is only between ~8-23%. (Schumacher, Hindler et. al, 2020, “Benchmarking R&D success rates of leading pharmaceutical companies: an empirical analysis of FDA approvals”)
[In some cases you don’t need all trial phases, e.g. for generics or biosimilars, also in some cases phases can be combined to speed up development]