In the CLINICAL DEVELOPMENT [05] dashboard you will see the Clinical Development Project Portfolio (left) with all clinical projects in your project-pipeline. For each project you see details like Project-Name, Project-Number, Therapeutic Area, Compound Type, details on the Clinical Development Phase (Phase I, Phase II, Phase III) including estimated costs and funding information. On the right you see the most important departments in Clinical Development: Clinical Operations, Biostatistics, Clinical Data Management and Medical. By clicking of one of these departments you can directly enter the respective department dashboard (see also Human Resources [08]).
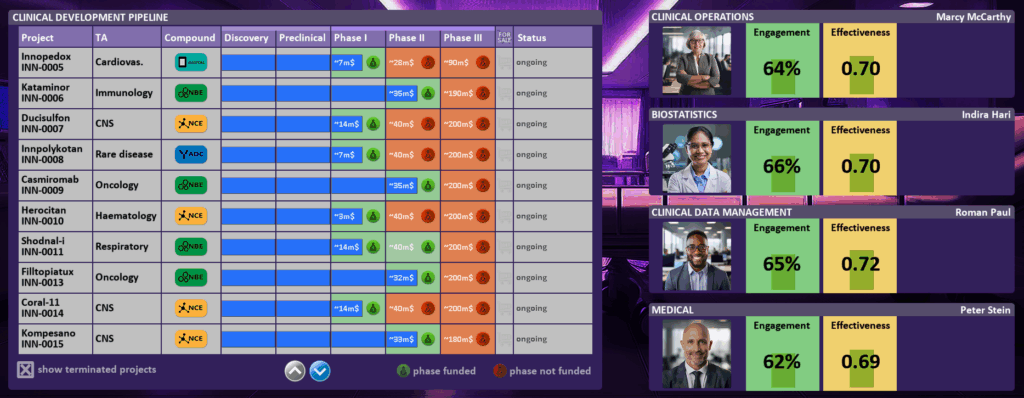
Clinical Development Project-Pipeline (left): By clicking on the phase of a project, you can allocate a budget to it or deallocate budget from it. If you allocate money/cash, this money is taken from CASH and added to your PLANNED money (and vice versa).
Control your view of projects using the “show terminated projects” checkbox. Later in the game, it is recommended to uncheck this option to focus only on active projects and maintain efficiency.

In the above example we see a CLINICAL DEVELOPMENT project Kataminor (INN-0006), in TA Immunology, NBE, that is currently in Phase II and Phase II is funded (estimated costs until end of phase II still 35m$). Phase III is currently not funded and would cost approx. 190m$.
If you want to mark a project “FOR SALE” you can just click on the “FOR SALE” column on the project to mark it for sale. Another click/touch unmarks it. You can then check under BUSINESS DEVELOPMENT [10] if there are some other companies interested to buy your project.
If you need to make a project decision or want more details on the individual project you need to click on the individual project (e.g. in the status column or at project name, TA or Compound) and you will get directly to a detailed project dashboard:
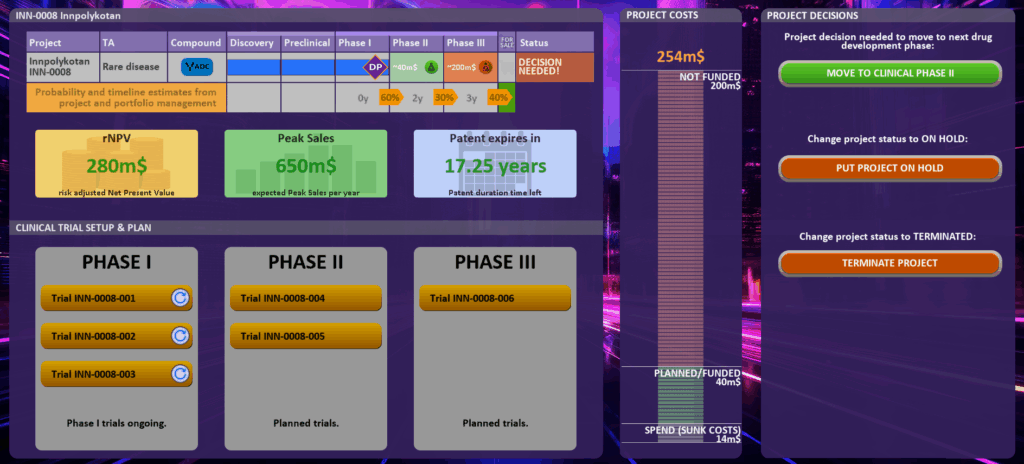
In the project dashboard you see the project details including the current estimated success probabilities (by your portfolio management function) for reaching the next development phases. In the above example Phase II will take 2 years and the estimated probability for success in Clinical Phase II is 30%.
Additional Information shown in project details:
- rNPV (risk adjusted Net Present Value) – see explanation in Appendix
- Peak Sales (potential) – see explanation in Appendix
- Patent duration – see explanation in Appendix
…and some additional details on the clinical program and the clinical studies conducted, in progress or needed.
In the middle of this dashboard you also see the project costs: sunk costs (already spent money), planned/funded money and the amount of project costs that is not funded yet (maximal cost you need to invest in addition to move to submission).
In the “PROJECT DECSIONS” area (on the right) you can move the project to the next R&D phase (if the project is currently successfully at a decision point) or decide to put the project on hold or terminate the project.
| Clinical Development is the process of bringing new medicines, devices or treatments through scientific experiments to the market. It involves conducting clinical trials on humans to evaluate the effectiveness and safety of these new products. Typically, there are 3 phases until approval (I, II, III) with an increasing number of participants. Phase IV is after product launch. Usually, the primary goals of a trial in a clinical phase are: Phase I: Dose-ranging on healthy volunteers or patients for safety Phase II: Testing of drug on participants to assess efficacy and side effects Phase III: Testing of drug on participants to assess efficacy, effectiveness and safety (Phase IV: post marketing surveillance in public) – this phase is excluded from this simulation If the drug or product successfully passes through Phase I, II, and III, it will get approved by the regulatory authorities. |
Tutorial Video: Clinical Development