In the REGULATORY & COMMERCIAL [06] dashboard you have access to your regulatory & commercial project/product portfolio. For each project/product you see if it is still in submission (Status Column), or sale details if it is on the market. On the right you see the departments: Regulatory Affairs and Commercial. By clicking on the departments you get to the detailed departmental dashboards (see HUMAN RESOURCES [08])
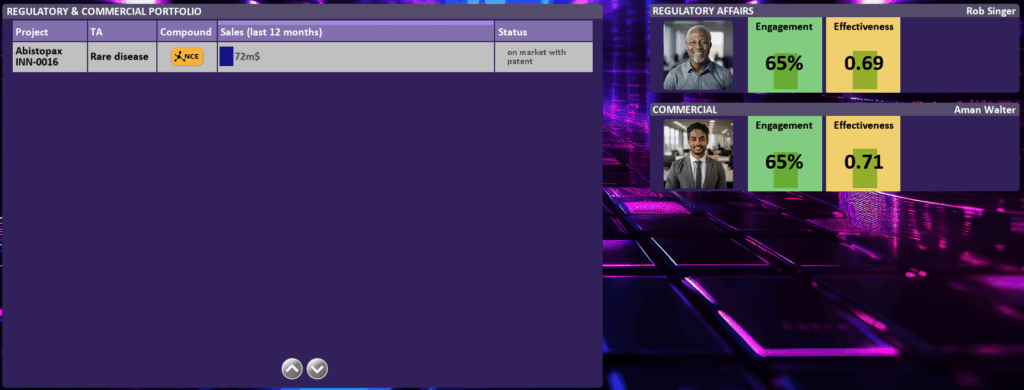
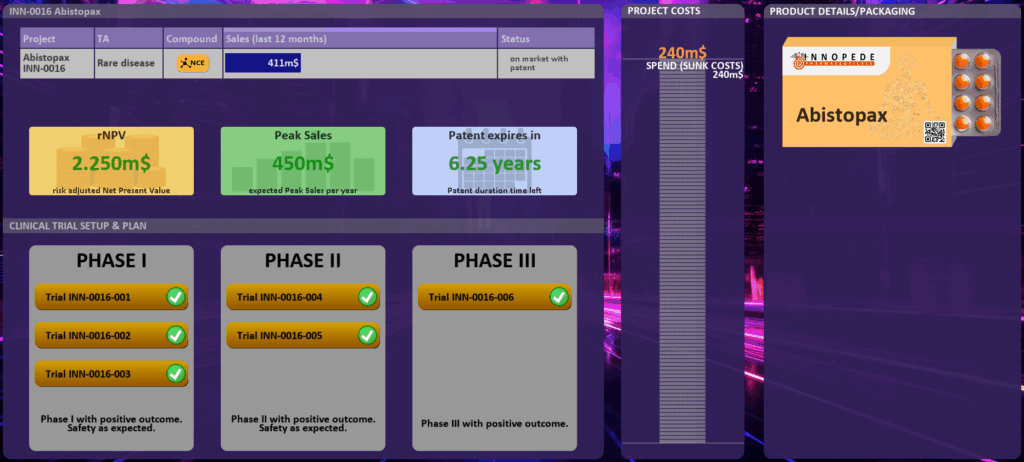
By clicking on the project, you can open the project details as described in project dashboard (in FUNCTIONAL DASHBOARDS [3]). If the project is approved and your drug/medication/digital solution is on the market, you will also see the product packaging.
| Regulatory Affairs plays a crucial role in ensuring that drug development and commercialization process comply with all relevant regulations and guidelines. It defines the regulatory strategy, manages the regulatory submissions to the regulatory authorities (Investigational New Drug Applications (IND), Clinical Trial Applications (CTA), New Drug Applications (NDA) and ensures the submissions adhere to the regulatory standards and required data formats. Regulatory Affairs also manages regular communication with the regulatory agencies, manages the approval of labeling and packaging materials and supports life cycle management. The commercial organization is responsible for market research, branding, sales and distribution. |
This game simplifies the regulatory and commercial aspects to focus on project management and learning about various functions.