Regulatory Affairs
Regulatory Affairs plays a crucial role in ensuring that drug development and commercialization process comply with all relevant regulations and guidelines. It defines the regulatory strategy, manages the regulatory submissions to the regulatory authorities (Investigational New Drug Applications (IND), Clinical Trial Applications (CTA), New Drug Applications (NDA) and ensures the submissions adhere to the regulatory standards and required data formats. Regulatory Affairs also manages regular communication with the regulatory agencies, manages the approval of labeling and packaging materials and supports life cycle management.
Regulatory Submission and Data Formats
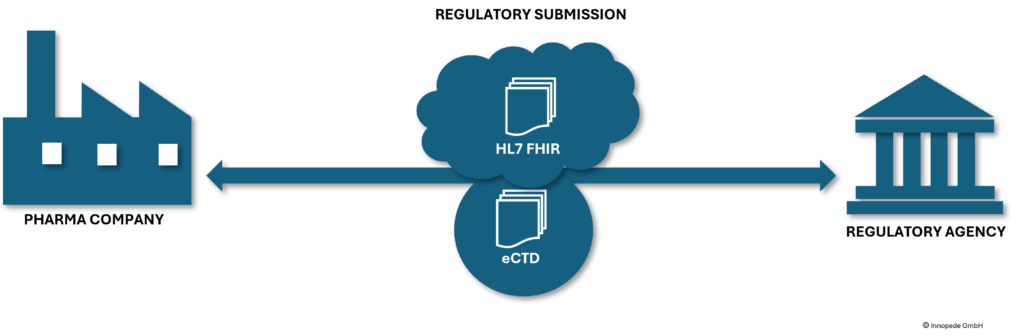
Submission is done in eCTD (electronic Common Technical Document) format to the health authorities. In future this will be in a central cloud. The data formats for clinical trial data are defined by CDISC ((Clinical Data Interchange Standards Consortium).
- SDTM (Study Data Tabulation Model)
- ADaM (Analysis Data Model)
- CDASH (Clinical Data Acquisition Standards Harmonization)
Commercial
The commercial organization is responsible for market research, branding, sales and distribution.